Shopping Cart [more]
Index of Products
Information
- 9PTK Titration Instructional Videos
- Automatic Rinse Tank Controls
- Boiler and Cooling Towers
- Circuit Board Cleanliness Testing
- Deionized Water
- Environmental Applications
- Fountain Solutions
- Frequently Asked Questions
- Glossary of Terms
- Hemodialysis Applications
- Horticulture
- Hydroponics
- Material Safety Data Sheets
- Operation Manuals / Instructions
- Oxidation Reduction Potential (ORP)/Redox
- Pool and Spa
- Product Data Sheets
- Reverse Osmosis
- Technical Reference: Handheld pH Instruments
- Textile Manufacturing
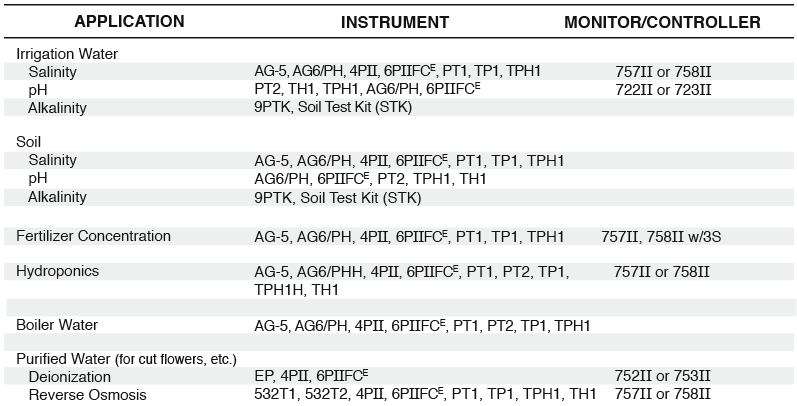
Horticulture
WHY ARE TESTS SO IMPORTANT?
Modern growing practices include scientific evaluations of soil, water, fertilizers, diseases, etc. While some tests are best performed by a laboratory, others can be easily conducted on location, saving time and money. Three tests in particular, EC, pH, and ALKALINITY, can reveal valuable information about water quality, soil salinity, and fertilizer concentration. Myron L® Company’s portable AGRI-METERS™ provide you with a simple, fast, and accurate means of testing these parameters.
WHAT IS ELECTRICAL CONDUCTIVITY (EC)?
EC is the measurement of a solution’s ability to conduct an electrical current. For horticultural applications, the unit of measure is often expressed as millimhos. Absolutely pure water is actually a poor electrical conductor. It is the substances (or electrolytes) dissolved in the water which determine how conductive the solution will be.
Therefore, EC can be an excellent indicator of:
- Water quality
- Soil salinity
- Fertilizer concentration
EC AND WATER QUALITY
The quality of irrigation water is one of the most critical factors influencing your growing operation. It is important to have a complete water analysis performed on a regular basis. Environmental conditions such as drought, changing seasons, heavy rainfall, etc., can cause the concentrations of dissolved salts in your water to vary significantly. These dissolved salts (i.e. calcium, sodium, etc.) can directly affect your plants’ health and, over time, render even the best soil useless.
You can monitor your overall water quality by testing its electrical conductivity with a Myron L® AGRI-METER™. The higher the EC, the more salts are dissolved in your water. By comparing your EC with previous readings, you can tell if any dramatic changes have occurred. Nutrient deficiencies are possible when water is too pure (low EC) or if the relative concentrations of some nutrients are unbalanced (i.e. calcium/magnesium). On the other hand, nutrient toxicities or osmotic interferences can also be traced to water quality. Water EC of even one millimho or below can cause problems. High EC readings of more than two millimhos can suggest serious problems, and special cultural procedures may be required.
EC AND SOIL SALINITY
“Water, water, everywhere, but not a drop to drink” is an old saying that applies to your plants when the soil salinity becomes too high. Salts from irrigation water and fertilizers tend to accumulate in your soil or growing media. High soil salinity disrupts the normal osmotic balance in plant roots. In severe cases a plant will become dehydrated even when the soil is wet. Symptoms of high soil salinity include: leaf chlorosis and necrosis, leaf drop, root death, nutrient deficiency symptoms, and wilting. All too often these symptoms are not recognized as being caused by soluble salts in the growing media. Sampling your soil and testing the EC of an extract can reveal important information about a soil’s suitability and your crop’s health.
Samples should be representative of different depths and locations. An easy-to-perform extract method is available with the Myron L® Soil Test Kit. A 2:1 or 5:1 water-to-soil ratio is made using the small vials provided. Soil test labs often use a method that calls for testing the EC of an extract from a thicker slurry. Therefore, you may see higher soil EC readings from a lab. It is important to standardize your sampling, extract, and testing methods. This will keep the difference between lab and field testing to a predictable factor.
EC AND FERTILIZER CONCENTRATION
You know how important fertilizer is to your plants, but do you know how accurate your fertilizer dosage is? Relying on traditional proportional methods is risky to plants and can waste expensive fertilizer. Improperly mixed fertilizer or a malfunctioning injector can lead to less than optimal results or even a disastrous loss of crops. Many fertilizer companies now recommend using a simple EC test to verify correct fertilizer concentrations. Many growers check their fertilizer injectors on a weekly basis, or they use a continuous EC monitor.
Fertilizer companies and suppliers often can provide a chart relating EC to parts per million concentrations of their various fertilizers. If one is not available for the fertilizer you use, carefully make some stock solutions at commonly used strengths and test their EC. This will give you a data base for future reference.
To test the EC of fertilizer solutions:
- Test and record the EC of the water to be mixed with the fertilizer.
- Test the conductivity of the fertilizer and water mixture.
- Subtract the water conductivity determined in #1 above.
- The resulting figure is an accurate indication of how much fertilizer is present (a higher conductivity means more fertilizer).
Important note: Interpretation of results differs from formula to formula and even among manufacturers of the same formula. Obtain the proper EC charts from the fertilizer company.
Myron L® Company manufactures both portable and inline instrumentation to make your fertilizer monitoring easy. Myron L® AGRI-METERS™, AG-5 and AG6/PH, TH1, waterproof TECHPRO II™ models TP1, TPH1 and TH1, and waterproof ULTRAMETER II™ models 4P and 6PFCE are handheld instruments which make fertilizer testing as simple as filling a cup and pushing a button.
The Myron L® 750 Series II™ EC Monitor/controllers can be used to continuously monitor your fertilizer concentration. Their “alarm” relay circuit acts as a safeguard in a fertilizer injection system or even as the main controller for your injector. A 0-10 VDC output for chart recorders or PLC (SCADA) input is standard on all monitor/controller models.
IMPORTANCE OF pH
pH, the measure of acidity or basicity, should be included in any soil or water test. It is well documented that growing media pH is critical to successful plant growth. This is especially true for new soilless mixes and hydroponics. pH affects the roots’ ability to absorb many plant nutrients. Examples include iron and manganese, which are insoluble at high pHs and toxic at low pHs. pH also directly affects the health of necessary micro-organisms in soil.
The effectiveness of pesticides and growth regulators can be severely limited by spray water pH that is either too low or too high.
ALKALINITY
It is important to note that testing the pH of irrigation water reveals only part of the story. Testing water alkalinity (bicarbonates and carbonates) is much more important than generally recognized. Alkalinity dictates how much influence the water’s pH will have on your soil and nutrient availability. In addition, alkalinity has a very great effect on the ease or difficulty of reducing the pH of water.
Other products and companies referred to herein are trademarks or registered trademarks of their respective companies.
**Prices, images and specifications subject to change without notice.**


For your convenience, we accept these credit cards: