Shopping Cart [more]
Index of Products
Information
- 9PTK Titration Instructional Videos
- Automatic Rinse Tank Controls
- Boiler and Cooling Towers
- Circuit Board Cleanliness Testing
- Deionized Water
- Environmental Applications
- Fountain Solutions
- Frequently Asked Questions
- Glossary of Terms
- Hemodialysis Applications
- Horticulture
- Hydroponics
- Material Safety Data Sheets
- Operation Manuals / Instructions
- Oxidation Reduction Potential (ORP)/Redox
- Pool and Spa
- Product Data Sheets
- Reverse Osmosis
- Technical Reference: Handheld pH Instruments
- Textile Manufacturing
Oxidation Reduction Potential (ORP)/Redox
WHAT IS ORP?
Oxidation Reduction Potential or Redox is the activity or strength of oxidizers and reducers in relation to their concentration. Oxidizers accept electrons, reducers lose electrons. Examples of oxidizers are: chlorine, hydrogen peroxide, bromine, ozone, and chlorine dioxide. Examples of reducers are sodium sulfite, sodium bisulfate and hydrogen sulfide. Like acidity and alkalinity, the increase of one is at the expense of the other.
A single voltage is called the Oxidation-Reduction Potential, where a positive voltage shows a solution attracting electrons (oxidizing agent). For instance, chlorinated water will show a positive ORP value whereas sodium sulfite (a reducing agent) loses electrons and will show a negative ORP value.
ORP is measured in millivolts (mV), with no correction for solution temperature. Like pH, it is not a measurement of concentration directly, but of activity level. In a solution of only one active component, ORP indicates concentration. As with pH, a very dilute solution will take time to accumulate a measurable charge.
An ORP sensor uses a small platinum surface to accumulate charge without reacting chemically. That charge is measured relative to the solution, so the solution “ground” voltage comes from the reference junction - the same type used by a pH sensor.
HISTORY OF ORP
ORP electrodes were first studied at Harvard University in 1936. These studies showed a strong correlation of ORP and bacterial activity. These tests were confirmed by studies on drinking water and swimming pools in other areas of the world. In 1971 ORP (700 mV) was adopted by the World Health Organization (WHO) as a standard for drinking water. In 1982 the German Standards Agency adopted the ORP (750 mV) for public pools and in 1988 the National Swimming Pool Institute adopted ORP (650 mV) for public spas.
WHERE IS ORP USED?
As you can tell by the previous paragraphs, ORP is used for drinking water, swimming pools and spas. However, ORP is also used for cooling tower disinfection, groundwater remediation, bleaching, cyanide destruction, chrome reductions, metal etching, fruit and vegetable disinfection and dechlorination.
In test after test on poliovirus, E. coli, and other organisms, a direct correlation between ORP and the rate of inactivation was determined. It is, therefore, possible to select an individual ORP value, expressed in millivolts, at which a predictable level of disinfection will be achieved and sustained regardless of variations in either oxidant demand or oxidant concentration. Thus, individual ORP targets, expressed in millivolts, can be determined for each application, which will result in completely reliable disinfection of pathogens, oxidation of organics, etc. Any level of oxidation for any purpose can be related to a single ORP number which, if maintained, will provide utterly consistent results at the lowest possible dosage.*
* Courtesy of Siemens Stranco Products.
WHY USE ORP?
ORP is a convenient measure of the oxidizer’s or reducer’s ability to perform a chemical task. ORP is not only valid over a wide pH range, but it is also a rugged electrochemical test, which can easily be accomplished using in-line and handheld instrumentation. It is by far a more consistent and reliable measurement than say chlorine alone.
LIMITATIONS FOR ORP
As with all testing, ORP has certain limitations. The speed of response is directly related to the exchange current density which is derived from concentration, the oxidation reduction system, and the electrode. If the ORP of a sample is similar to the ORP of the electrode, the speed will be diminished.
Carryover is also a possible problem when checking strong oxidizers or reducers, and rinsing well will help greatly.
Although a better indicator of bactericidal activity, ORP cannot be used as a direct indicator of the residual of an oxidizer due to the effect of pH and temperature on the reading. ORP can be correlated to a system by checking the oxidizer or reducer in a steady state system with a wet test, and measuring pH. If the system stays within the confines of this steady state parameter (usually maintained by inline or continuous control), a good correlation can be made. The best recommendation for ORP is to use wet tests, and over three test periods correlate the ORP values to those test parameters.
FREE CHLORINE CONVERSION USING ORP
The most ubiquitous and cost-effective sanitizing agent used in disinfection systems is chlorine. When chlorine is used as the sanitizer, free chlorine measurements are required to ensure residual levels high enough for ongoing bactericidal activity. Myron L® digital handheld instrumentation accurately converts ORP measurements to free chlorine based on the understanding of the concentrations of the forms of free chlorine at a given pH and temperature. The conversion is accurate when chlorine is the only oxidizing/reducing agent in solution and pH is stable between 5 and 9. This pH range fits most applications because pH is usually maintained such that the most effective form of free chlorine, hypochlorous acid, exists in the greatest concentration with respect to other variables such as human tolerance.
MYRON L® INSTRUMENTS
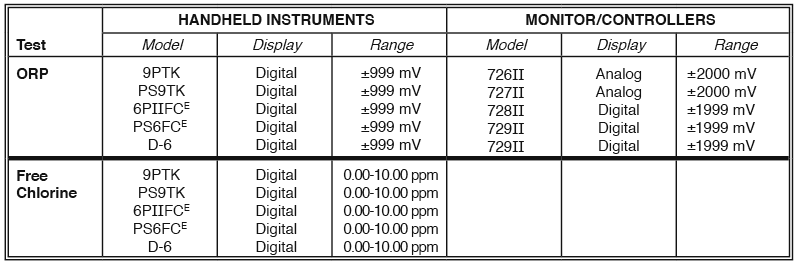
Myron L® Company offers a variety of handheld instruments and in-line Monitor/controllers that may be used to measure, monitor and/or control ORP. The following table lists some of the model numbers for measuring, monitoring, and/or controlling ORP. The Ultrameter III™ 9PTK, Ultrameter II™6PFCE, POOLPRO™ PS9TK and PS6FCE and D-6 DIGITAL DIALYSATE METER™ are multi-parameter handheld instruments with ORP and free chlorine measuring capabilities. These instruments also have the capability to measure conductivity, TDS, resistivity, pH, mineral/salt concentration and temperature, making them the preferred instruments for all water treatment professionals. The 720 Series II Monitor/controllers are an excellent choice for continuous in-line measurements.
Other products and companies referred to herein are trademarks or registered trademarks of their respective companies.
**Prices, images and specifications subject to change without notice.**


For your convenience, we accept these credit cards: